Abstract
BACKGROUND: The transcription factor B cell CLL/lymphoma 11B (BCL11B) is indispensable for T lineage development of lymphoid progenitors. Tonic signaling of chimeric antigen receptors (CARs) during early phases of in vitro generation of hematopoietic stem cell (HSC)-derived lymphoid progenitors leads to suppressed T cell development and acquisition of NK cell-like properties (CAR-induced killer cells, termed CARiK cells) by suppression of BCL11B. Co-transplantation of CARiK cells into hematopoietic stem cell recipients mediates strong antigen-specific anti-leukemic effects. Here we investigated whether CRISPR/Cas9-based editing of Bcl11b would allow the generation of CARiK cells using clinically more relevant CARs w/o tonic signaling activity.
METHODS: Antigen binders against murine CD123 were identified using phage display technology. High affinity binders were cloned into a 2nd generation murine CAR backbone (CD28CD3zeta configuration) and comparatively assessed for expression strength, functionality, specificity, and the lack of tonic signaling activity. Aiming for an all in one vector approach, a sgRNA sequence against Bcl11b was added to the construct for CRISPR-mediated gene disruption. Transduced murine LSK cells were then differentiated into lymphoid CARiK progenitors using the OP9-DLI feeder layer system. Double edited progenitors (8 x 106) and respective controls were co-transplanted into leukemia-bearing murine stem cell recipients. BCL11B suppression was assessed by Western-blotting, correlated with the appearance of a CARiK phenotype, and finally tested for in vivo functionality.
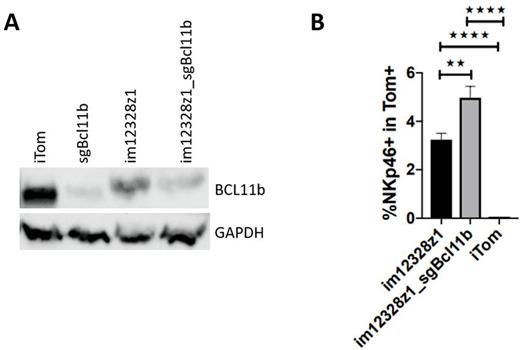
RESULTS: A suitable CD123 CAR of high expression strength, specificity, and devoid of tonic signaling was generated. Despite the lack of tonic signaling, early stimulation via CD123 in culture was sufficient for some degree of NK-directed developmental shift. The degree of fratricide was limited. Early Bcl11b disruption allowed for further lymphoid cell differentiation. Bcl11b editing only let to complete BCL11B suppression as did the combined all in one vector construct (Figure 1A). CAR transduction w/o Bcl11b editing resulted in incomplete BCL11B suppression. After transduction with the lentiviral all in one construct, the strongest developmental NK cell shift was observed (Figure 1B). After co-transplantation of CARiK cells up to 100% leukemia free survival was achieved. Low expression strength of CD123 on physiologic hematopoiesis allowed for undisturbed recovery of myelopoiesis as compared to controls.
CONCLUSION:Bcl11b editing of hematopoietic stem cell-derived CAR-engineered lymphoid progenitors allows the generation of a NK cell-like cell product with considerable anti-tumor activity. Genetic stability remains to be determined.
Figure 1: Natural killer-like cell differentiation of HSC-derived mCD123 CAR-engineered lymphoid progenitors can be enforced by Bcl11b editing. (A) Partial suppression of BCL11B by early CAR-signaling and finally complete disruption by Bcl11b editing as shown by Western Blotting (iTom = neg. control). (B) The extent of BCL11B suppression correlates positively with the amount of NKp46+ cells within the CAR-expressing gate in flow cytometry analysis (n=3). (B) Statistics was performed using Student's t test (2 tailed). Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal